What is Glutamic Acid Residue and Its Importance in Proteins?
glutamic acid residue is a vital component in the structure of proteins. This amino acid plays a crucial role in determining protein function and stability. As a negatively charged residue, it can facilitate various biochemical interactions.
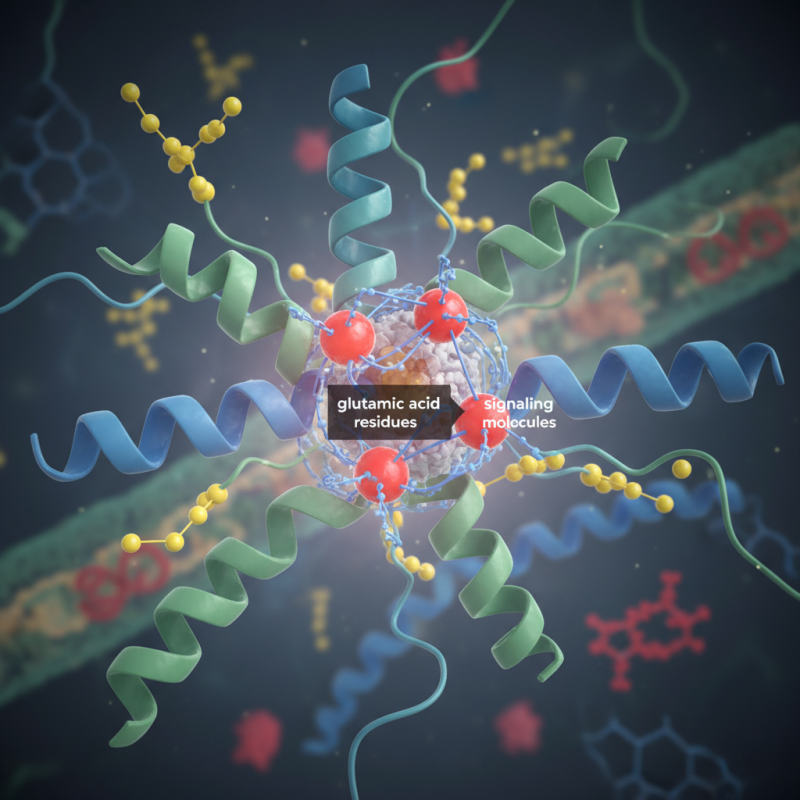
In cellular processes, glutamic acid residue often acts as a signaling molecule. Its ability to form hydrogen bonds greatly influences protein folding. This folding is essential for a protein’s biological activity. Without these residues, proteins may not function correctly, leading to cellular dysfunction.
Many proteins contain multiple glutamic acid residues. The presence of these residues can enhance enzyme activity or create binding sites for other molecules. Researchers continue to explore their importance in health and disease. Understanding these residues may unveil new therapeutic targets. However, the complexities of their interactions often pose challenges. There is still much to learn about their broader implications in biology.
What Is Glutamic Acid Residue and Its Role in Protein Structure?
Glutamic acid residue plays a critical role in protein structure. This amino acid is known for its carboxyl group, which carries a negative charge at physiological pH. This charge is essential for forming salt bridges and stabilizing protein structures. Proteins often adopt complex shapes, and glutamic acid helps maintain these conformations.
In enzyme active sites, glutamic acid residues can facilitate chemical reactions. They can donate or accept protons during catalysis, making them vital for enzyme function. However, an excess of glutamic acid could disrupt overall protein structure. If present in high amounts, it might lead to misfolding or aggregation.
The importance of these residues cannot be overstated. They are also involved in biochemical signaling. Glutamic acid can interact with other molecules, influencing cellular processes. Yet, there are cases where mutations in glutamic acid residues lead to diseases. Studying these residues offers insights into how proteins function and how errors can arise. Understanding their role is crucial for advancing protein chemistry.
Biochemical Properties of Glutamic Acid Residues in Proteins
Glutamic acid residues play a crucial role in protein chemistry. These amino acids are negatively charged at physiological pH, making them vital for protein stability and function. Reports indicate that about 12% of natural proteins contain glutamic acid. Its carboxyl group can form hydrogen bonds, influencing protein structure.
In enzymatic reactions, glutamic acid often acts as a proton donor or acceptor. This property is essential for catalyzing biochemical reactions. Many enzymes require glutamic acid for activity, demonstrating its importance in metabolic pathways. Research shows that mutations in glutamic acid residues can lead to enzyme dysfunction. This impact emphasizes the need for careful analysis of these residues in protein engineering.
However, not all glutamic acid residues are beneficial. Some can create steric hindrance in protein folding. Over-accumulation of these residues might destabilize protein structures. The balance between sufficient glutamic acid and excessive amounts must be addressed in protein design. This complexity calls for more research to fully understand their dual role in proteins.
The Role of Glutamic Acid in Enzyme Activity and Function
Glutamic acid is an essential amino acid found in many proteins. Its unique side chain plays a critical role in the structure and function of proteins. In enzymatic reactions, glutamic acid acts as a key player. It helps facilitate the binding of substrates and stabilizes reaction intermediates. This is crucial for many biochemical processes.
In enzymes, glutamic acid often participates in the catalytic site. It can donate or accept protons, influencing the reaction's speed. This versatility allows enzymes to operate efficiently under various conditions. However, not all glutamic acid residues function identically. Their positioning within the protein structure can affect their interaction with other molecules.
Understanding these nuances is vital. A single change in the glutamic acid residue can alter enzyme activity drastically. This unpredictability poses challenges for researchers. Investigating these discrepancies could lead to important discoveries. Exploring the multifaceted roles of glutamic acid in enzymes remains an ongoing journey, rich with potential for new insights.
Glutamic Acid Residue's Impact on Protein Folding and Stability
Glutamic acid residues play a crucial role in protein folding and stability. These residues are negatively charged, which helps in stabilizing protein structures. Their presence influences how proteins interact with one another. When glutamic acid is positioned properly, it can form hydrogen bonds and ionic interactions with nearby amino acids. This connectivity is vital for the protein’s three-dimensional shape.
However, not all situations are ideal. Sometimes, the placement of glutamic acid can lead to misfolding. This issue can cause the protein to lose its function. Misfolded proteins may even aggregate, leading to diseases. Researchers often explore these structural challenges. They strive to understand how small changes in glutamic acid locations can impact overall stability. The complexity of protein interactions underscores the need for further investigation. There’s still much to learn about these dynamics. Further reflection on these aspects could lead to advancements in biotechnology.
Impact of Glutamic Acid Residue on Protein Stability
Clinical Implications of Glutamic Acid in Neurotransmission and Health
Glutamic acid plays a crucial role in neurotransmission. This amino acid acts as a primary excitatory neurotransmitter in the brain. Glutamate is involved in sending signals between nerve cells. It is essential for cognitive functions, such as learning and memory. However, too much glutamate can lead to toxicity. This excess is linked to neurodegenerative diseases. Understanding this balance is vital for maintaining brain health.
In the context of health, glutamic acid serves various functions. It supports muscle metabolism and helps synthesize proteins. Adequate levels may improve mood and cognitive clarity. However, its impact on health is complex. Some individuals may be sensitive to glutamate in foods. This sensitivity can lead to headaches or digestive issues. More research is needed to understand these individual differences thoroughly.
Nutritional sources of glutamic acid include meat, fish, and certain vegetables. Diet plays a significant role in regulating glutamate levels. Reflecting on personal dietary habits may reveal areas for improvement. Individuals should consider how their intake affects their well-being. This aspect of health deserves more attention and exploration.